Need an assist with Medical Devices?
Improving patient outcomes and saving lives is the ultimate goal of any medical device manufacturer and every medical device launch. If only the process were as simple as designing an innovative new device and sending it out into the marketplace!
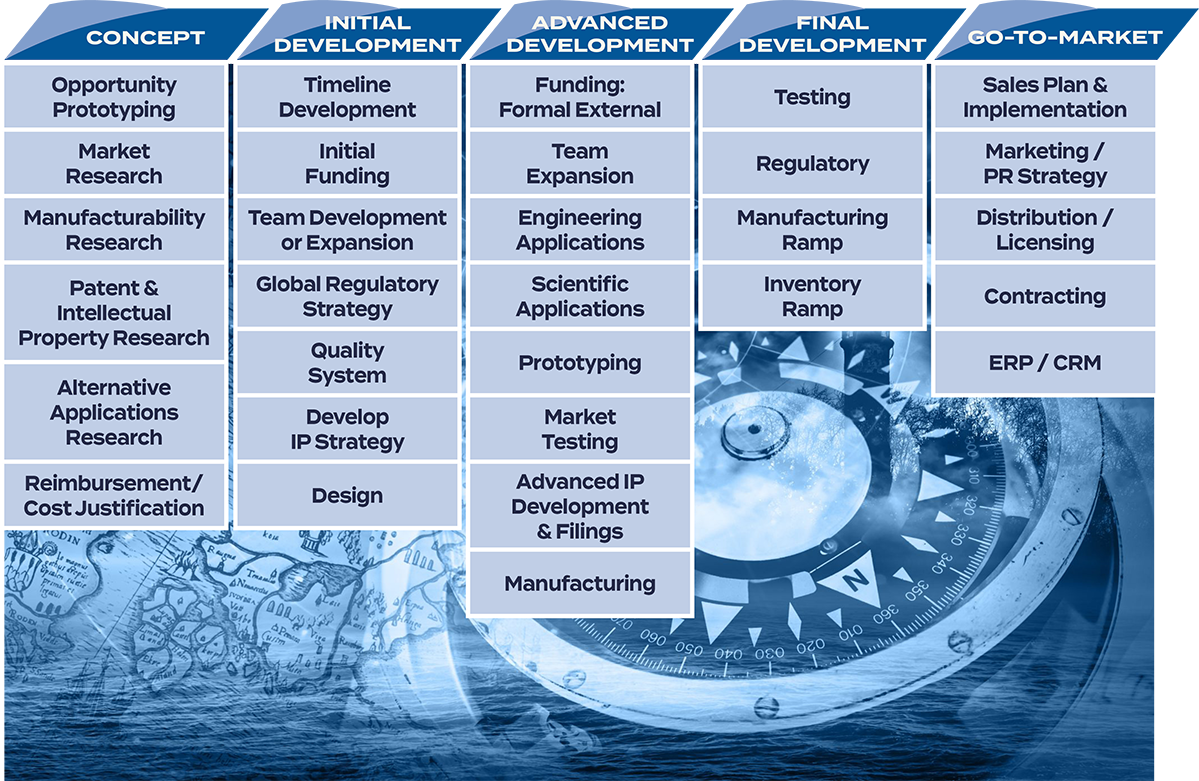
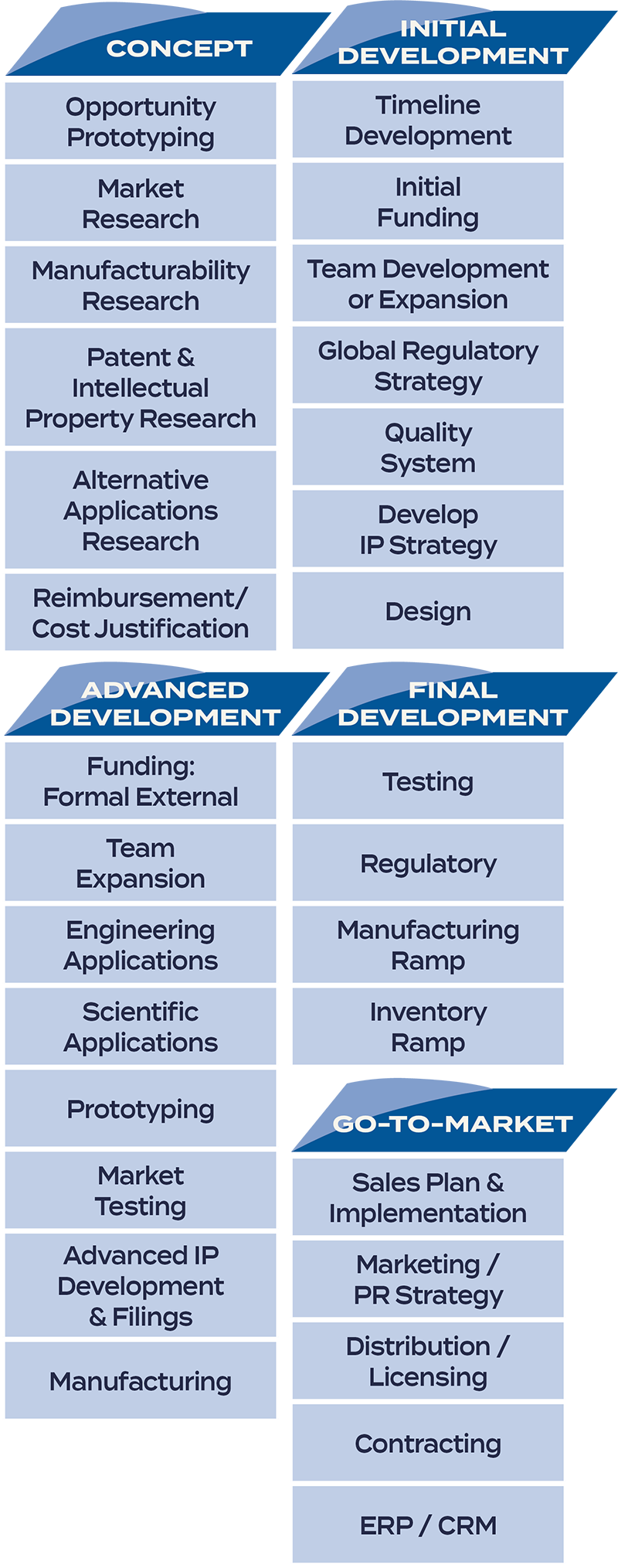
Instead, United States, European and specific country regulations present significant hurdles to even the best ideas for a medical device. We know because we have taken new technology concepts from design to prototype to completed product. And guided companies through the high-stakes maze of medical device development and clearances. We’ve navigated the often conflicting market, regulatory, and user requests that inform medical device development from the start. We know what questions to ask at the concept phase that will impact quality systems, design efforts, testing, regulatory clearances, and auditing and marketing requirements much further down the road.
Trust our market expertise.
Let us guide you through this complicated maze of rules and regulations – always ensuring safety, efficacy and ease of use for the physician or clinician. The Yar Group’s significant medical device development and go-to-market experience helps our clients create strategies to ensure compliance, and prepare for key FDA, CE mark, Australian TGA and other regulatory agency applications.
With our guidance, you can:
- Speed your time to market
- Meet or surpass regulations both domestic and abroad
- Create a quality-driven approach to design, manufacturing, testing, and launch
- Understand marketing and labeling requirements
- Identify market issues with user-evaluation studies
Reach the finish line.
The Yar Group is a powerful partner to navigate the confusing and high-stakes road to medical device clearances. Quality-driven strategies and processes mean faster results for medical device manufacturing companies.
Ready to focus on what you do best – innovation? Contact us to see how we can work together. 505.805.5002.